Globally, over $5 billion is spent per year on cancer research. Yet, for those of us unlucky enough to receive a diagnosis, most cancers doctors will offer the same standard treatment options that they have offered for well over half a century: surgery, chemotherapy, and/or radiotherapy. Despite improvements to all three options over the decades, they each still carry risks of damaging normal tissue or failing to eradicate the cancer.
Nanoparticles might provide a way to dramatically reduce these risks and improve patient outcomes due to their numerous advantageous properties. For example, their small size means that they can passively accumulate in tumors due to what is known as the enhanced permeation and retention effect. And their ample surface area can be functionalized with ligands to actively direct them to desired locations in the body.
As a result, various types of nanoparticles are being investigated for guiding surgical resection of tumors, delivering chemotherapeutic agents more effectively, and enhancing the therapeutic efficacy of radiation-based treatments. They may even open the door to the creation of new therapeutics using nanomaterial properties themselves.
However, there remain significant roadblocks to nanoparticle-based therapy. “Some magnetic nanoparticles have been used during surgery where they wanted to cut out a glioblastoma, but only directly to tumor cells,” explains Joanna Depciuch of the Institute of Nuclear Physics, Polish Academy of Sciences, Poland. “They are afraid to inject these nanoparticles, because they aren’t sure where these nanoparticles will accumulate and they are concerned that ions, which can interact with our blood, can cause a huge toxic effect.”
Depciuch is laser focused on solving these challenges, particularly in regard to the application of gold nanoparticles, known to be lethal to cancer cells, in anticancer therapies. Her BioPhotonics plenary, “Application of nanoparticles in anticancer combination therapies,” on Sunday night presented fascinating new insights her research has uncovered.
Previous to this year, it was thought that the smaller the gold nanoparticles are that are applied to cancer cells, the better. The logic behind this was that smaller nanoparticles would find it easier to sneak into the cancer cell core, where they could cause maximum damage and ultimately cell death.

Joanna Depciuch of the Institute of Nuclear Physics, Polish Academy of Sciences. Credit: Institute of Nuclear Physics Polish Academy of Sciences, Krakow.
Depciuch decided to study this in more detail using a holotomographic microscope. This advanced instrument — the first to be purchased in Poland — combines tomography with an optical microscope to enable visualization of the interaction between cells and nanoparticles based on differences in refractive index.
In more detail, a beam of electromagnetic radiation is carefully calibrated to ensure its energy does not interfere with cellular metabolism. As the beam scans the cells, it generates holographic cross-sections that reveal variations in the refractive index. The differences in light refraction between the cytoplasm, cell membrane, and nucleus enable the reconstruction of a detailed 3D image, capturing both the cell’s external structure and its internal composition.
Crucially, the resulting holotomographic image not only offers 3D information on nanoparticle accumulation in these cells with nanometric resolution, from all sides simultaneously and practically in real time, it does so without using fluorophores (fluorescent molecules often used to tag cells or other molecules). “Normally, we know that the fluorophores cause some toxic effects,” reveals Depciuch. “But all changes that we observed in the morphology of the cells were caused by the nanoparticles, not by the nanoparticles and fluorophores.”
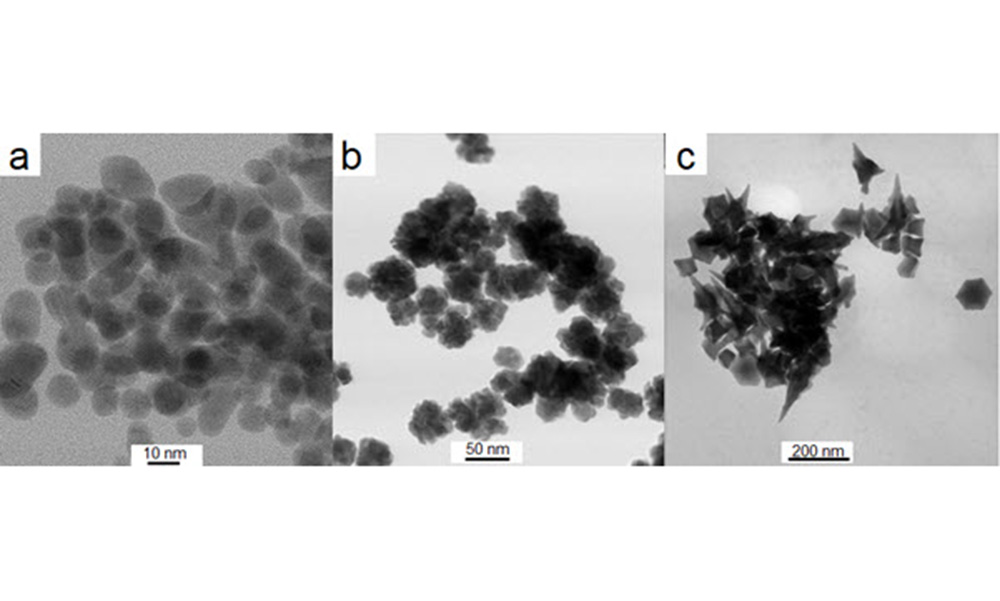
With this clarity, Depciuch and colleagues were able to paint a more nuanced picture: “We obtained information that the accumulation and the absorption of these nanoparticles depend on the shape of the nanoparticles, size of the nanoparticles, and also the type of cancer cells.” For example, tiny spherical nanoparticles, 10 nanometers in size, had almost no effect on either a glioma or colon cancer cell line, whereas much larger (200 nanometers) star-shaped nanoparticles resulted in high mortality in just 20 minutes, due to their sharp tips perforating the cell membranes to the point that the cells could not cope with repairing the increasing damage, leading to programmed cell death.
Using these results, the researchers built a theoretical model of nanoparticle deposition. This model can already be used by other researchers to quickly determine the uptake of a certain size and shape of nanoparticle by cancer cells over a given period of time.
“We have created a model whose parameters are only the shape and size of the nanoparticles and the different kinds of cells, and these are just glioblastoma and colon cancer cells,” confirms Depciuch. “But I want to extend these experimental results to create a model where we put all physical properties of the nanoparticles (chemical composition, zeta potential, size, shape, and, for example, concentration of these nanoparticles in different kind of cells) and use it to try to analyze which kind of nanoparticles we should use for which kind of cancer cells.”
Such a model will be of huge benefit to the community, allowing researchers to immediately eliminate a huge swathe of property combinations that they would otherwise have to eliminate via time-consuming and costly experimental verification. What is more, it will enable researchers to focus on the most promising experiments in which the nanoparticles will be particularly well absorbed by selected cancer cells, while maintaining low or zero toxicity to healthy cells in the patient’s other organs.
So far, with the Polish team having conducted experiments on only three cell lines — two glioma and one colon — the model’s applicability is limited. Current work is aiming to design experiments to include further parameters, such as the chemical composition of the particles and further tumor types, in order to build the model Depciuch envisions.
At the same time as building a picture of what types of nanoparticles work best on which cancers, Depciuch is exploring the best ways to apply gold nanoparticles. For example, star-shaped nanoparticles appear to be ideal if applied directly to a given tumor, but more caution must be taken if directed to the cells via the blood for chemotherapy. “The stability of the nanoparticles in the blood is different than when we put them in, for example, a water solution,” she says. “And we know that in the blood these nanoparticles can lead to gold ions being present, which are toxic to organs.”
Depciuch is also exploring the use of different nanoparticle sizes and shapes in novel therapeutic strategies. One is photothermal therapy, a treatment for certain cancers such as skin cancer. “First, we choose a nanoparticle concentration that is non-toxic,” she says. “Then we can focus this laser only where we have the cancer cells, which irradiates these nanoparticles to activate the toxic effect.” Depciuch says that spherical gold nanoparticles best absorb ~525 nanometer wavelength light, which is of no use for phototherapy. But the nanoparticles she has engineered with rod-like shapes best absorb light around 800 nanometers (near-infrared light) making them ideal candidates for use in photothermal therapy.
The message she hopes the audience took away with them from her BioPhotonics plenary? “I hope that everybody will see the promising future of nanoparticles in medicine, but also that we should be very, very, very careful about using nanoparticles now, when we know, I think, less than we should.”
Benjamin Skuse is a science and technology writer with a passion for physics and mathematics whose work has appeared in major popular science outlets.This article originally appeared in the 2025 SPIE Photonics West Show Daily.